Before delving into “how is cement manufactured?” it’s critical to understand the fundamentals. First, let’s learn about what cement is, the composition of cement, the main properties of cement, different types of cement, and the key ingredients used in the cement manufacturing process.
Table of Contents
What is cement?
Cement is a generic term that refers to a variety of silicates, aluminates, and ferrites derived from limestone and clay. Cement is a dark green, coarse powder composed mostly of Tricalcium Silicate (3CaO.SiO2/Ca3SiO5), Dicalcium Silicate (2CaO.SiO2/Ca2SiO4), Tricalcium Aluminate (3CaO.Al2O3/Ca3Al2O6) and Tetracalcium Aluminferrite (4CaO.Al2O3.2Fe2O3). These compounds account for about 90% of total cement. The most abundant component of cement is Tricalcium Silicate.
What is the composition of cement?
The below graph provides the average composition of the commonly used Portland cement.
| Compound | Average Composition |
|---|---|
| CaO | 62% |
| SiO2 | 22% |
| Al2O3 | 7.5% |
| MgO | 2.5% |
| Fe2O3 | 2.5% |
| K2O | 1% |
| Na2O | 1% |
What are the main properties of cement?
- Density
The term “density” refers to the mass-to-volume ratio. That is, the amount of cement held in a unit volume. To fill a cubic meter of volume, about 28.8 cement bags are needed. As a result, the following procedure can be used to determine the concrete density:
Weight of 1 cement bag = 50kg
Therefore, weight of 28.8 cement bags = 50*28.8 = 1440kg
Hence the weight of cement in 1m3 = Density of cement = 1440kgm-3
The 1440kgm-3 value is called the bulk density, where the volume of voids is also included in the calculation of volume. If the volume of voids is not included, the density is referred to as the absolute density or packed density, which equals 3150kgm-3. The Specific Gravity of a substance is the density of the particular substance relative to the density of standard substance which is water (1000 kgm-3). Hence, the specific gravity of concrete can be calculated as (3150/1000) 3.15.
The “Density of Hydraulic Cement (ASTM C188 & AASHTO T133 )” tests are the industry standards for determining density. There the test is performed using a standard Le Chatelier flask. The specific gravity of cement can also be tested in accordance with ASTM C 188.
2. Fineness
As a reactive powder, one of the most critical aspects of cement is its particle size distribution, which determines the overall surface area. The fineness of cement is a measure of the particle size of the cement and is stated in terms of the cement’s specific surface area. The rate at which cement hydrates is determined by its fineness, which is proportional to its particle size. The setting time, compressive strength, shrinkage, and permeability of the cement are all dependent on its fineness.
There are three standard tests for determining the specific surface area in the cement industry: Blaine (ASTM C204), Wagner (ASTM C115), and sieve residue-45 μm sieve (ASTM C430).
Blaine operates on the concept that the permeability of a bed of tiny particles is proportionate to their fineness. Thus, the test is a measurement of the air flow rate through a bed of cement particles with one side at vacuum and the other at atmospheric pressure. This is derived directly from the Kozeny-Carman approximation theory, which is based on the packing of mono-size spherical particles.
Wagner test is also known as the turbidimeter fitness test. It is used to determine the turbidity of a cement suspension suspended in kerosene. This technique is rarely utilized nowadays.
The sieve residue test (45 μm) is used to determine the quantity of cement retained on a calibrated sieve as a proxy for the proportion of particles larger than a certain size. Further reading here.
3. Composition
Final product obtained by proportioning the required raw materials bears the below average composition.
| Compound | Average Composition |
|---|---|
| Lime (CaO) | 62% |
| Silica (SiO2) | 22% |
| Alumina (Al2O3) | 7.5% |
| Alumina (Al2O3) | 7.5% |
| Iron Oxide (Fe2O3) | 2.5% |
| Magnesium Oxide (MgO) | 2.5% |
| Sulphur Trioxide (SO3) | 1.25% |
| Alkalies (from clay) | traces |
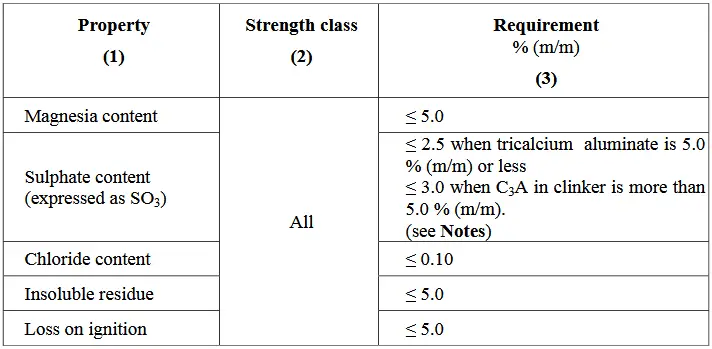
However, based on the Sri Lankan Standards the chemical composition of cement may be determined using either the wet chemistry method specified in SLS ISO 29581-1, or the X-ray fluorescence (XRF) methodology described in SLS ISO 29581-2. Chemical criteria for all OPC strength classes should be in accordance with those indicated in the following table. ASTM C144 and AASHTO T105 are the industry standards for “Chemical Analysis of Hydraulic Cement.”
4. Consistency
The Standard Terminology for Hydraulic Cement (ASTM C 219) defines “normal consistency” as “a degree of plasticity in a hydraulic-cement paste that is suitable for testing as determined by a specified method.” Normal consistency is calculated as the mass of water necessary to create this plasticity divided by the mass of hydraulic cement, represented as a percentage. ASTM C187 and AASHTO T129 are the standard test techniques for determining the normal consistency of hydraulic cement.
5. Inter-ground materials
Inter-grounding or blending is the process of mixing two or more inorganic ingredients (at least one of which is not Portland cement or Portland cement clinker) that contribute to the cement’s strength-gaining capabilities either individually or in combination. Fly ash, slag, limestone, and gypsum are all examples of inter-ground materials.
To get the desired concrete compressive strength, limestone is mixed with cement. Fly-ash contributes to achieving the required compressive strength of cement and lowering the cost of manufacturing. Portland cement is blended with 25%±2% Class F fly ash in IPF cement. Slag, a byproduct of iron and steel manufacturing, aids in the production of high-strength concrete (HSC). IS Cement or Slag Cement being a hydraulic cement comprised mostly of granulated blast-furnace slag and Portland cement, or hydrated lime, or both, with the slag ingredient being at least 70% of the slag cement’s mass.
When a trace of gypsum is added to cement, it retards the setting process by reacting with Tricalcium Aluminate (3CaO.Al2O3) to generate a double crystalline Sulphoaluminate (3CaO.Al2O3.3CaSO4.3H2O). A modest quantity of gypsum may significantly boost the strength of cement. However, when the gypsum level exceeds 5%, the structure expands slowly.
The standard for Blended Hydraulic Cement is ASTM C595.
6. Strength gain characteristics
At a laboratory temperature of 27±2 0C, the compressive strength of cement is evaluated using mortar prisms in accordance with SLS ISO 679. Essentially, two types of strengths are evaluated: standard and early. The compressive strength of mortar prisms at 28 days is used to estimate the standard strength of cement. Cement’s early strength is assessed by the compressive strength of mortar prisms after two or seven days. For each class of standard strength in the table below, two classes of early strength are included: one with average early strength, denoted by N, and one with high early strength, denoted by R.
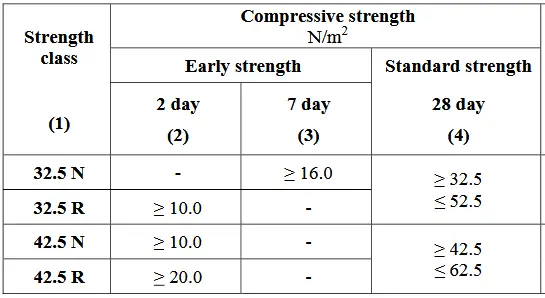
The relevant standard test methods are the “Compressive Strength of Hydraulic Cement Mortar- ASTM C109 & AASHTO T 106” and “Tensile Strength of Hydraulic Cement Mortars- ASTM C190(withdrawn) & AASHTO T132”.
7. Soundness
Soundness is critical to ensuring that the cement does not fluctuate significantly in volume after setting. Certain cements have been seen to expand significantly after setting, disrupting the set and hardened mass. When such cement is employed, major difficulties with construction durability will arise. It is critical to test the soundness of cement to guarantee that it does not exhibit substantial future expansion. When evaluated for soundness using the technique provided in SLS ISO 9597, the cement should not expand more than 10 mm.
The soundness of cement can also be determined using the Le- Chatelier principle. The “Standard Test Method for Autoclave Expansion of Hydraulic Cement- ASTM C 151 & AASHTO T 107“ are the standard tests for determining the cement soundness.
8. Setting time
When water is added to cement, it sets and hardens. This setting time varies according to a variety of conditions, including the fineness of the cement, the cement-water ratio, the chemical content such as availability of different salts, the atmospheric conditions, and the use of admixtures.
The initial setting time of cement used in building should not be too short and the final setting time should not be too long. The time interval between adding water to the cement and the onset of plasticity in the paste is referred to as the early setting time.
The final setting time is the time interval between when the water is introduced to the cement and when the cement paste entirely loses its plasticity and reaches a firmness adequate to withstand a certain defined load. After the concrete is placed, the initial setting takes 30–45 minutes, and the final setting takes around 10 hours. Concrete starts to harden visibly during the early setting process. At the end of the setting process, the cement hardens well and becomes capable of sustaining a load.
When it comes to concrete transportation, placement, and curing, the initial setting time is crucial to know. Delay of hydration or hardening is also accomplished by the use of early setting time. The final setting time is used for the safe removal of formwork from the construction site.
The time required for the first setting of cement paste of standard consistency, as measured by the procedure given in SLS ISO 9597. The “Time Setting of Hydraulic Cement by Vicat Needle (ASTM C 191 & AASTO T 131)” and the “Time Setting of Hydraulic Cement by Gillmore Needle (ASTM C 266) & AASHTO T154)” tests are the industry standards for determining the setting time of hydraulic cement.
9. Heat of Hydration
Hydration is a sequence of irreversible chemical reactions between hydraulic cement and water that results in the formation of new compounds, the majority of which have strength-producing properties. With time, the formation of hydration compounds results in a decrease in workability (stiffening), a solidification (setting), and an increase in strength (hardening). Hydration is an exothermic process that produces heat. The heat released is referred to as the heat of hydration. The hydration procedure is repeated until no heat and moisture are present in the cement.
Chemical composition:
The heat generated is mostly due to the interaction between the cement components C3S (3CaO.SiO2), C2S (2CaO.SiO2), C3A (3CaO.Al2O3), and C4AF (4CaO.Al2O3.2Fe2O3). At high temperatures, the oxides included in these basic compounds react with one another to generate complex compounds. Due to the fact that R.H. Bogue identified these compounds, they are often referred to as Bogue’s compounds or Bogue’s composition.
The C3S reaction generates additional heat required for hydration. It provides the initial strength (first seven-day strength), which makes cement with a higher C3S content appropriate for cold weather concreting.
C2S generates less heat as a result of hydration. C2S hydrates at a very low rate and begins to increase the temperature gradually. This increase in temperature prolongs the duration of the hydration, allowing the internal heat to remain at its maximum for an extended period of time. This, however, has no effect on the strength of the concrete. C2S steadily hydrates and hardens after 7 days, providing a significant portion of the final strength.
C3A reacts spontaneously with water. This leads to immediate stiffening of the cement paste, referred to as “flash setting.” However, the hydrated C3A does not contribute to the strength of concrete. C4AF undergoes rapid hydration and does not affect the concrete strength.
In addition to the chemical composition there are several factors which effect the heat of hydration in cement. They are the fineness, water cement ratio, temperature at placing, curing temperature, environmental temperature, cement type and content, sulfate content, effect of Supplementary Cementous Materials (SCM) and admixtures, etc.
Fineness:
With increasing fineness, the rate of cement hydration accelerates. The surface area of finer cement particles is greater than that of coarse cement particles. This results in increased interaction between water and the tiny cement particles during mixing, resulting in rapid hydration.
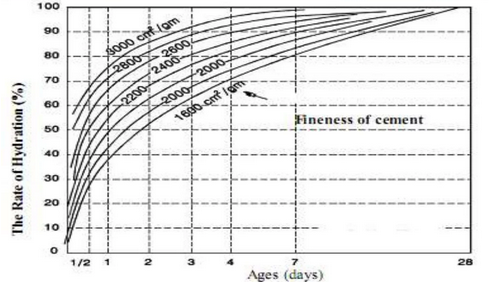
Cement type and content:
The heat of hydration varies with the cement types specified in BS EN 197-1 as follows.
| Heat of hydration (7 days J/g) | Cement type | Description |
|---|---|---|
| 350 | Ⅰ | Portland cement |
| 265 | Ⅱ | Portland composite cement |
| 370 | Ⅲ | Blast furnace cement |
| 235 | Ⅳ | Pozzolanic cement |
| 310 | Ⅴ | Composite cement |
Cement is the primary component of concrete that generates hydration heat. When mixtures with high cement content are hydrated, they generate a lot of heat. According to ACI 211.1, the standard practice for choosing proportions for normal, heavyweight, and mass concrete, the thermal gradient in concrete generated by 50 kg of cement per 1m3 is around 5 to 7 °C within 18 to 72 hours.
W/C ratio:
Heat of hydration gets increased with the w/c ratio. When the water-to-cement ratio is reduced, the amount of water available for cement hydration is reduced. As a consequence, the quantity of heat lost during hydration will be decreased. This, however, will leave some cement paste dried, resulting in internal tension and poor bonding in the concrete.
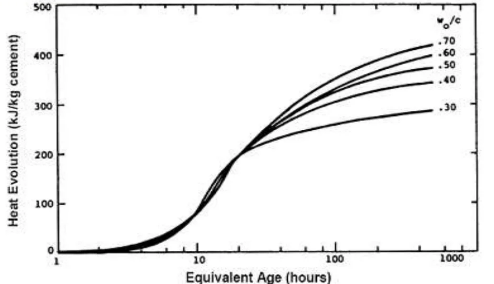
Temperatures of placing, curing and environmental temperature:
The temperature of fresh concrete is a critical component in determining the hydration temperature. In general, if the temperature of placing concrete is high, the hydration temperature will also be high. Thermal fractures in concrete may occur at elevated temperatures of hydration. This can be overcome by the use of ice and chilled water for mixing, carrying out mixing at night and aggregate cooling.
Hot weather conditions accelerate the hydration process, resulting in a rapid setting. In cold weather, the hydration reactions generate heat. It is capable of providing some degree of self-protection against an external temperature that would otherwise result in freezing or a slowed hardening process.
Curing cement at higher temperatures increases the early age hydration rate and promotes heat of hydration. This is beneficial for concrete hardening. However, this will result in a significant decrease in long-term compressive strength.
Use of SCM and admixtures:
The use of supplementary cement converts CH to C-S-H (gel). By delaying setting and strength gain, these materials often retard hydration and increase workability. They will prevent heat peaks and increase heat production. Certain additives, such as accelerators, may boost hydration and provide a quick set, generating a higher heat of hydration. However, this can be overcome by incorporating a retarder such as fly ash into the concrete. Retarders decrease the rate of hydration, providing more time for heat to be dissipated into the surrounding environment.
Methods to control the heat of hydration
- Pre-cooling and post-cooling
- Controlling cement content in mix-design.
- Using low-heat cement
- Using chilled water and ice for mixing
- Night-concreting
- Using retarders such as fly ash
- Using steel/aluminum formwork systems.
The BS-EN 197-1 standard classifies 27 cement types in the family of common cement into five main groups based on their major constituents.
- CEM Ⅰ – Portland Cement
- CEM Ⅱ – Portland composite cement
- CEM Ⅲ- Blast furnace cement
- CEM Ⅳ- Pozzolanic cement
- CEM Ⅴ – Composite cement
There are 3 basic types of cement based on the setting time.
| Cement | Time taken for 1st stage of setting | Time taken for 2nd stage of setting | Use |
|---|---|---|---|
| Quick setting | 10-20 mins | 10-30 mins | Under water constructions |
| Medium setting | 10-30 mins | 3 mins to 5 hrs. | Ordinary masonry work |
| Slow setting | Not less than 30 mins | 3-7 hrs. | Road construction, Bridges and Dam construction |
In Sri Lanka, there are three main types of cement that are utilized.
- OPC – Ordinary Portland Cement
- PPC – Portland Pozzolana Cement
- PLC – Portland Limestone Cement
Raw materials of cement production
Three main raw materials are used.
- Calcareous component
- Argillaceous material containing Silica
- Material to delay the cement hardening process.
| Calcareous component | Argillaceous component | Agent to delay setting |
|---|---|---|
| CaCO3 Limestone Marble Chalk Coral | SiO2+Al2O3 Clay Slate Shale Blast furnace slag | Gypsum (CaSO4.2H2O) |
Manufacture of Cement
Two methods are involved in the cement production.
- Dry process
This method is used in Sri Lanka. Here, raw materials such as natural cement rock and limestone, shale, or slate combinations are crushed, dried, and combined in a 1:5 ratio before being put into a rotary kiln. Subsequently, between 600°C and 1500°C, the dry mixture is burned to generate clinker, which will then be reduced to a fine powder.
The rotary kiln consists of 4 parts
- Preheating zone – Water gets removed.
- Calcining zone – Oxidization of organic compounds, Decomposition of CaCO3 and clay (1000°C)
- Clinkering zone – Formation of Calcium Silicates and Calcium Aluminates with the reaction of free oxides (1300°C-1500°C)
- Cooling zone – Cooling clinker and grounding with 4-5% of Gypsum.
- Wet process
This is utilized in the manufacture of high-quality cement. This is the original technique. The raw ingredients (limestone and clay) are crushed and combined with water to make a slurry, which is then heated in a rotary oven. This procedure is divided into four stages: slurry preparation, calcination, cooling, grinding, and storage. The slurry undergoes following steps of changes inside the kiln.
1. Loss of moisture
When slurry reaches the area of 300°C the water gets evaporated.
2. Limestone decomposition
Around 1400°C-1500°C limestone gets decomposed to CaO and CO2.
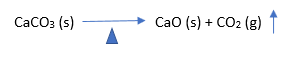
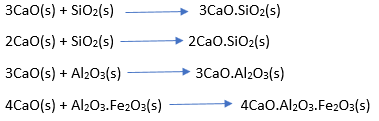
3. Sintering
At kiln lower end lime reacts with clay at 1400°C-1500°C to form Calcium Silicates and Calcium Aluminates.
4. On the way to the chimney the gases from kiln passes through a dust chamber.
5. Setting or Hardening of cement. Setting takes place in 2 stages.
- First stage setting
During this period, two significant processes occur.
1. Tricalcium Aluminate is hydrated to produce multiple crystalline hydrates. The most prevalent of these is 3CaO.SiO2.6H2O.
2. Tricalcium Silicate hydrolysis results in the formation of hydrated lime and Dicalcium Silicate.
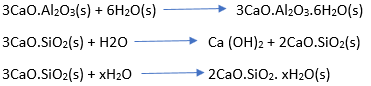
The hydrates are water insoluble and contribute to the hardening of the mass. Calcium Silicate precipitates a gelatinous substance on the unreacted cement particles.
- Second stage setting
During this stage, the gelatinous film of hydrated Dicalcium Silicate gradually loses water, partially by rehydration of previously treated material and also through evaporation. Thus, the Dicalcium Silicate deposit eventually hardens and binds other materials together, such as brick fragments and stones.
Use of Gypsum:
Gypsum retards the setting of cement. Gypsum reacts with Tricalcium Aluminate to form a double crystalline Sulphoaluminate.

A modest amount of gypsum contributes to the strength of the cement to a certain level. However, if the gypsum concentration is greater than 5%, the structure will expand slowly.